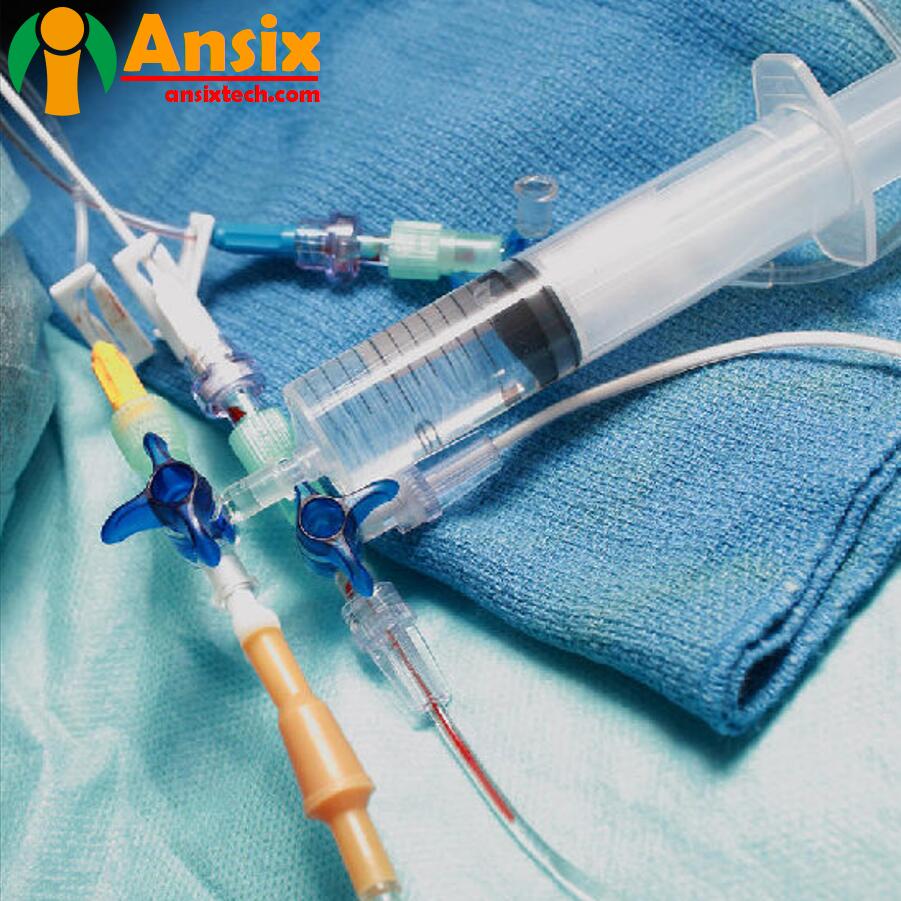
Medical Consumables & Disposables Manufacturers
description
- Secondly, medical equipment is also one of the important application areas of medical injection molds. Medical equipment includes various medical monitors, surgical instruments, medical electronic equipment, etc. These devices often require complex structures and functions to meet medical operation and treatment needs. Medical injection molds can produce various complex-shaped plastic parts according to the design requirements of the equipment, such as casings, connectors, sensors, etc., providing key support for the production of medical equipment.In addition, medical injection molds can also be used in the production of medical consumables. Medical consumables include various medical plastic products, such as surgical masks, masks, gloves, dressings, etc. These consumables usually need to have good breathability, waterproofness and comfort to ensure the smooth progress of medical operations. Medical injection molds can design appropriate mold structures and injection molding processes based on the characteristics and requirements of consumables to produce high-quality medical consumable products.
- Finally, medical injection molds can also be used in the repair and renewal of medical devices and equipment. With the continuous development of medical technology, medical devices and equipment are being updated faster and faster. Sometimes, just replacing a few plastic parts can make an old device look like new again. Medical injection molds can be copied and produced based on the parts of old equipment to achieve equipment repair and update and extend the service life of the equipment.In short, the medical injection mold application program uses injection mold technology to produce medical devices and medical equipment, providing important support and guarantee for the medical industry. With the continuous advancement of medical technology, medical injection mold application solutions will become increasingly diversified and refined, making greater contributions to the development and progress of the medical industry.We constantly expand our medical plastic injection molding capabilities, and high-speed German injection molding machines are equipped in our ISO 8 clean room. The fine screw of our injection molding machine can be accurately measured to 0.02g, clean room molding is also beneficial for the production of transparent items for medical use.
Services

- Plastic Medical Consumables Design
Plastic medical consumables design refers to the design process of using plastic materials to manufacture medical devices and consumables. Plastic materials are widely used in the medical field because it has many advantages, such as corrosion resistance, easy cleaning, low cost, etc. The design of plastic medical consumables needs to consider the following aspects:Material selection: Choose plastic materials suitable for medical applications, such as polyethylene, polypropylene, polyvinyl chloride, etc. Materials should have good biocompatibility and chemical resistance.Structural design: Design a reasonable structure based on the functional requirements of medical devices and consumables. Taking into account factors such as ease of use, safety, reliability, and ease of cleaning.Manufacturing process: Select the appropriate manufacturing process according to the design requirements, such as injection molding, blow molding, extrusion, etc. Ensure product quality and performance.Standard compliance: Ensure that the designed medical consumables comply with relevant regulations and standard requirements, such as ISO 13485 quality management system and FDA certification, etc.Ergonomics: Taking into account the user experience of medical staff and patients, design products that conform to ergonomic principles, such as handle designs that conform to human hand shapes, and instrument shapes that conform to human body curves, etc.The design of plastic medical consumables needs to comprehensively consider factors such as materials, structures, manufacturing processes, standard compliance, and ergonomics to ensure product quality and performance, while improving the use effect and user experience of medical devices and consumables.
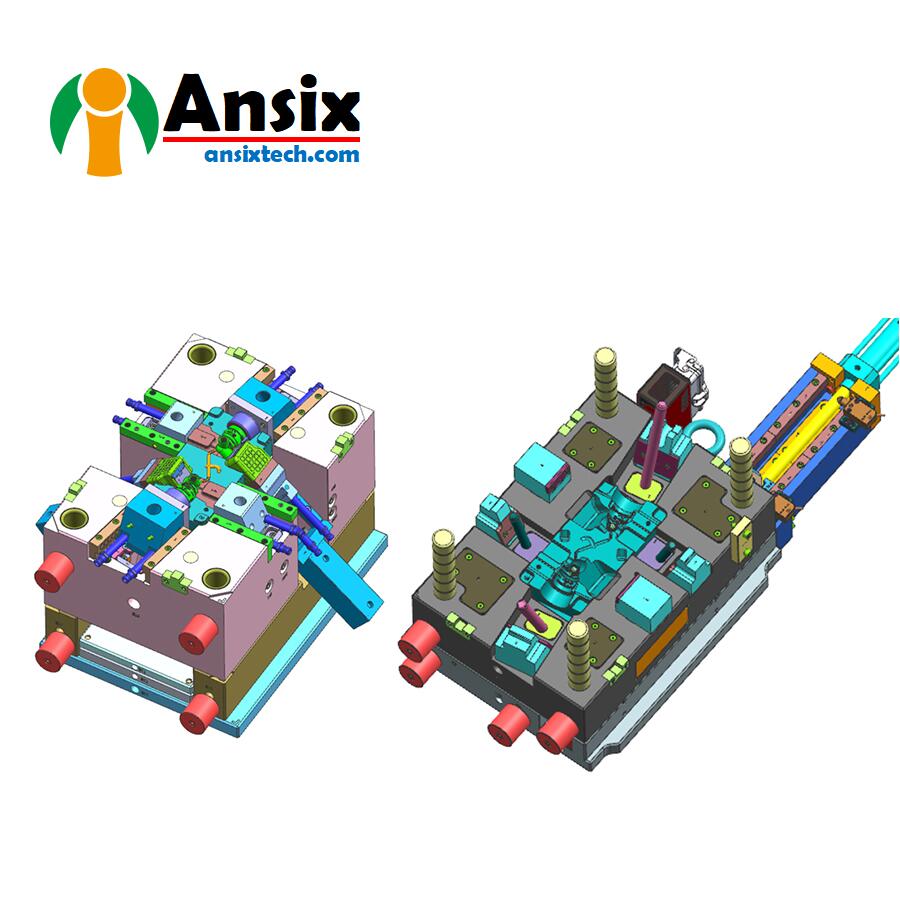
- Mold Design for Plastic Medical Consumable ItemsPlastic medical consumables mold design refers to molds designed for manufacturing plastic medical consumables. The goal of mold design is to achieve efficient, precise and reliable production of plastic products. Here are some key elements of plastic medical consumable mold design:Product design analysis: Analyze the product design of medical consumables to understand the shape, size, structure and other characteristics of the product to provide a basis for mold design.Material selection: Select suitable mold materials, such as high-quality steel, according to product requirements and production processes. The material should have high hardness, high wear resistance and high thermal conductivity to ensure the longevity and performance of the mold.Mold structure design: Design a reasonable mold structure according to the shape and size requirements of the product. Factors such as product formability, cooling performance and demoulding performance are taken into consideration. The mold structure should have sufficient rigidity and stability to ensure product accuracy and quality.Runner system design: Design a reasonable runner system to ensure that the plastic material can flow evenly and fill the mold cavity. The design of the flow channel should take into account factors such as the melting temperature, fluidity and cooling effect of the material.Cooling system design: Design an effective cooling system to speed up product cooling and improve production efficiency. The design of the cooling system should take into account the shape and size of the product, as well as the thermal conductivity of the material.Design of demoulding system: Design a reasonable demoulding system to ensure that the product can smoothly escape from the mold. The design of the demoulding system should take into account the shape and surface properties of the product, as well as the structure and materials of the mold.Mold processing technology: Choose appropriate processing technology, such as CNC machining, EDM, etc., to ensure the accuracy and quality of the mold.Plastic medical consumable mold design needs to comprehensively consider factors such as product design analysis, material selection, mold structure design, flow channel system design, cooling system design, demoulding system design and mold processing technology to ensure that the mold can meet the requirements of high quality and high efficiency. Plastic product production requirements.

- Material Consumables SelectionThe material selection of medical consumables is very important because it is directly related to the safety, biocompatibility and performance of the product. Here are some common medical consumable material options:Polyethylene (PE): Polyethylene is a commonly used medical consumable material with good corrosion resistance, chemical resistance and low cost. It is often used to make syringes, infusion bags, urine bags, etc.Polypropylene (PP): Polypropylene is a material with good biocompatibility and chemical resistance. It is often used to make infusion tubes, infusion sets, test tubes, etc.Polyvinyl chloride (PVC): Polyvinyl chloride is a commonly used medical consumable material with good corrosion resistance and chemical resistance. It is often used to make infusion tubes, urinary catheters, blood bags, etc.Polycarbonate (PC): Polycarbonate is a material with excellent transparency and impact resistance. It is often used to make infusion bottles, test tubes, syringes, etc.Polyester (PET): Polyester is a material with good transparency and chemical resistance. It is often used to make infusion bottles, test tubes, blood bags, etc.Polyamide (PA): Polyamide is a material with good heat resistance and chemical resistance. It is often used in the manufacture of high-temperature sterilization instruments, surgical instruments, etc.In addition to the above common materials, there are also some special medical consumable materials, such as silicone, polylactic acid (PLA), PEEK,PES,PBT,PEI,polylactic acid-polyethylene glycol copolymer (PLGA), etc. These materials have special properties and are suitable for specific medical applications.When selecting medical consumable materials, factors such as the functional requirements, biocompatibility, chemical resistance, heat resistance, and transparency of the product need to be considered. At the same time, it is also necessary to ensure that the selected materials comply with relevant regulations and standards, such as ISO 10993 biocompatibility testing and FDA certification.
- CNC Machining for Insert molding
CNC machining production technology for in-mold embedded parts involves the following aspects:Design and modeling: Use computer-aided design (CAD) software to design and model embedded parts. Determine the geometry, size and materials of embedded parts based on product needs and functional requirements.Processing path planning: Use computer-aided manufacturing (CAM) software to generate a processing path plan based on the CAD model embedded in the part. This includes cutting paths, cutting sequences, tool selection, cutting parameters, etc.Machine tool setup and preparation: According to the machining path planning, set the working parameters of the CNC machine tool, including fixtures, tools, workpiece coordinate systems, etc. Ensure that machine tools and cutting tools are prepared for machining operations.Processing operation: Load the processing path planning into the numerical control system of the CNC machine tool, and start the machine tool for processing operations. The CNC system controls each axial movement of the machine tool according to the instructions of the machining path planning to realize the cutting of embedded parts.Cutting parameter optimization: Optimize and adjust cutting parameters according to actual processing conditions. This includes the adjustment of cutting speed, feed speed, cutting depth and other parameters to improve processing efficiency and quality.Inspection and adjustment: During the processing, inspection and adjustment are carried out to ensure processing quality and accuracy. Use measuring instruments and tools to inspect embedded parts and make adjustments based on inspection results.Complete processing and cleaning: After a series of processing operations and adjustments, the CNC machining production of the embedded parts in the mold is completed. Clean and inspect the finished parts to ensure that their quality and accuracy meet requirements.When performing CNC machining production of in-mold embedded parts, attention needs to be paid to selecting appropriate tools, processing parameters and processing sequences to ensure the smooth progress of the processing process. At the same time, appropriate inspection and adjustment are also required to ensure processing quality and accuracy.The computerized production process is capable of handling even the tiniest variations in requests. We use CNC machining to handle big bulk medical supplies and maintain uniformity. This allows for precision manufacturing at different levels of production. 

- Medical Molding Method in Clean RoomWith each client order, various molding methods like micro molding, insert molding are operating at peak efficiency. The cleanroom here ensures sanitary production and reduces the risk of contamination throughout the production process.Medical product injection molding dust-free workshop has the following advantages:Product quality assurance: Dust-free workshops can effectively control the content of dust, bacteria and other pollutants in the air, reducing contamination to products. This can ensure that medical products are not affected by external contamination during the production process and improve product quality stability and consistency.Meet regulations and standards: The production of medical products usually needs to comply with strict regulations and standards, such as ISO 13485 quality management system and GMP (Good Manufacturing Practice). Dust-free workshops can provide a production environment that meets these requirements, ensuring product quality and safety to meet regulatory agency requirements.Reduce the risk of product contamination: Medical products are usually in direct contact with the human body. If the product is contaminated, it may cause risks to human health. Dust-free workshops can reduce the contamination of products by microorganisms and harmful substances in the external environment, reduce the risk of product contamination, and ensure the safety of patients.Improve production efficiency: Dust-free workshops can reduce pollution and interference during the production process and improve production efficiency. Dust-free workshops can reduce the defective rate of products, reduce downtime and maintenance costs in production, and improve the stability and productivity of the production line.Protect employee health: Dust-free workshops can provide a clean and safe working environment and reduce the risk of employees being exposed to harmful substances. This helps protect employee health and safety and improves employee job satisfaction and productivity.In summary, medical product injection molding dust-free workshops have the advantages of product quality assurance, meeting regulatory and standard requirements, reducing the risk of product contamination, improving production efficiency, and protecting employee health. These advantages are very important for the production of medical products to ensure product quality and safety and meet market demand and regulatory requirements.
- Secondary Operation for Injection Moulding
Injection molding secondary processing refers to the further processing and processing of the product after injection molding. These processes can improve the appearance, performance or functionality of a product to meet specific needs. Here are some common injection molding secondary processing methods:Cutting and trimming: Injection molded products are cut and trimmed to remove excess material or edges to make the size and appearance of the product more precise and neat.Grinding and polishing: Use grinding tools and polishing agents to grind and polish the surface of the product to remove surface defects, bumps and scratches, making the surface of the product smooth and bright.Spray coating and printing: Spray coating or printing on the product to add color, pattern or logo to the product. Spraying can use paint or coating, and printing can use screen printing, thermal transfer printing and other technologies.Assembly and packaging: Assemble multiple injection molded parts together to form a complete product. Assembly can use threaded connections, adhesives, mechanical connections, etc. After assembly is complete, the product is packaged to protect it from damage and contamination.Welding and heat treatment: Welding injection molded parts to achieve the connection of different parts. Welding can use hot melt welding, ultrasonic welding and other technologies. Heat treatment can change the physical properties and structure of a product through heating and cooling.Surface treatment: Special treatments are carried out on the surface of the product, such as sandblasting, electroplating, anodizing, etc., to increase the corrosion resistance, wear resistance and aesthetics of the product.Injection molding secondary processing can be customized according to the needs and requirements of the product to meet specific functional and appearance requirements. These processing methods can improve product quality, performance and market competitiveness. 
-
Injection Mold Solutions for Production Disposable Medical ItemsInjection molds for manufacturing disposable medical supplies are a vital part of the medical industry. The design and manufacturing of injection molds play a key role in ensuring the quality, safety and reliability of disposable medical supplies.First, mold design is key to manufacturing disposable medical supplies. Designers need to consider factors such as the product’s shape, size, functionality and material properties. The mold design should be able to achieve accurate molding of the product and ensure that the product’s appearance and performance meet the requirements.Secondly, material selection is critical to the longevity and performance of the mold. Disposable medical supplies often require mold materials with high hardness, high wear resistance and high thermal conductivity. Commonly used mold materials include high-quality steel, such as P20, H13, etc.Runner system design is key to the injection molding process. The runner system should ensure that the plastic material can flow evenly and fill the mold cavity. Reasonable flow channel design can reduce product defects and deformation.Cooling system design is crucial to the control of injection molding cycle and product quality. The cooling system should be able to cool the product quickly and evenly to improve production efficiency and the physical properties of the product.The design of the demoulding system plays an important role in the smooth release of the product from the mold. The demoulding system should ensure that the product can escape from the mold smoothly while avoiding damage and deformation of the product.The selection and control of mold processing technology are crucial to the accuracy and quality of the mold. Commonly used mold processing techniques include CNC machining, EDM, etc. Dimensions and surface quality need to be strictly controlled during processing to ensure the accuracy and reliability of the mold.Finally, mold debugging and optimization are key to ensuring that the mold can meet the requirements for high-quality, high-efficiency plastic product production. Mold trials and adjustments are required during the debugging process to ensure product quality and consistency.Manufacturing injection molds for disposable medical supplies requires strict compliance with relevant regulations and standards, such as ISO 13485 quality management system and FDA certification. Strict quality control and inspection are required during the mold manufacturing process to ensure that the quality and performance of the mold meet the requirements.Through reasonable mold design and manufacturing, the quality and safety of disposable medical supplies can be ensured and the needs of the medical industry can be met. The accuracy and reliability of injection molds are critical to the production of disposable medical supplies, ensuring product consistency and reliability and providing high-quality products to the medical industry.We can serve the medical business on a variety of accounts involving consumable materials. Our team manufactures china medical disposables using a variety of injection molding techniques. Overmolding, insert molding, and micro injection molding are among the services provided here.

- Medical Overmolding for AnsixTechMedical encapsulation molds are injection molds used to manufacture encapsulated products in the medical field. Rubber-coated products are usually composed of plastic substrates and rubber compounds. They are soft, durable, waterproof and comfortable. They are often used in medical devices, medical earplugs, medical catheters and other products.The design and manufacturing of medical overmolding molds need to consider the following key factors:Material selection: Medical encapsulation molds need to use wear-resistant and corrosion-resistant materials to ensure the life and performance of the mold. Commonly used mold materials include high-quality steel, such as P20, H13, etc.Mold structural design: The structural design of the medical encapsulated mold should take into account the shape, size and characteristics of the encapsulated product. The mold structure should be able to achieve precise molding of the product and ensure that the appearance and performance of the product meet the requirements.Runner system design: The design of the runner system plays an important role in the uniformity of the injection molding process and the stability of product quality. The runner system should ensure that the plastic material and rubber compound can flow evenly and fill the mold cavity.Design of rubber injection system: Medical rubber molding needs to consider the injection and curing process of rubber. The design of the rubber injection system should ensure that the rubber can be injected evenly into the mold cavity and solidified at the appropriate time and temperature.Design of the demoulding system: The design of the demoulding system plays an important role in the smooth release of the product from the mold. The demoulding system should ensure that the product can escape from the mold smoothly while avoiding damage and deformation of the product.Mold processing technology: The selection and control of mold processing technology are crucial to the accuracy and quality of the mold. Commonly used mold processing techniques include CNC machining, EDM, etc. Dimensions and surface quality need to be strictly controlled during processing to ensure the accuracy and reliability of the mold.The manufacturing of medical overmolding molds requires strict compliance with relevant regulations and standards, such as ISO 13485 quality management system and FDA certification. Strict quality control and inspection are required during the mold manufacturing process to ensure that the quality and performance of the mold meet the requirements.Through reasonable mold design and manufacturing, medical encapsulated molds can achieve high-quality, high-efficiency production of medical encapsulated products and meet the needs of the medical industry for encapsulated products.
- Insert molding for MedicalMedical insert injection molding refers to the manufacturing of inserts used in the medical field through injection molding. These inserts are usually used in products such as medical equipment, medical devices, and medical consumables to perform functions such as connection, transmission, and monitoring.The process of medical insert molding usually includes the following steps:Design the insert: Design the geometry, size and material of the insert based on the needs and functional requirements of the medical product. Consider the matching requirements between the insert and other components and the particularity of the use environment.Mold design: Design the injection mold according to the design requirements of the insert. Mold design should take into account the shape, size and characteristics of the insert, as well as the process requirements of injection molding.Material selection: Select the appropriate material for injection molding of the insert. Medical inserts usually require materials with high temperature resistance, corrosion resistance, wear resistance, etc., such as engineering plastics (such as ABS, PC, PA, etc.) or special materials (such as PEEK, PTFE, etc.).Mold manufacturing: According to the mold design, injection molds are manufactured. The mold manufacturing process includes mold processing, heat treatment, assembly and other steps to ensure the accuracy and quality of the mold.Injection molding: After heating and melting the selected material, the molten material is injected into the mold cavity through an injection molding machine, allowing it to cool and solidify. During the injection molding process, parameters such as temperature, pressure, and injection speed of the injection molding machine need to be controlled to ensure the quality and dimensional accuracy of the insert.Demolding and post-processing: After the injection molded insert is cooled and solidified, it is taken out from the mold and demoulded. After demoulding, post-processing processes such as deburring, edge trimming, and cleaning can be performed to improve the appearance and quality of the insert.Medical insert injection molding requires strict compliance with relevant regulations and standards, such as ISO 13485 quality management system and FDA certification. At the same time, strict quality control and inspection are also required to ensure that the quality and performance of the inserts meet the requirements.Through medical insert injection molding, high-quality, high-efficiency production of medical products can be achieved to meet the needs of the medical industry for inserts. Injection molding technology can realize the manufacturing of inserts with complex shapes. It also has the advantages of high production efficiency and low cost, and is suitable for mass production.The experts are known for providing intelligent and efficient injection molding solutions. The medical insert molding is done with the use of advanced equipment for high-precision medical molds. We also provide surgical disposable products through.


- Medical Micro injection mold
The manufacturing of medical micro-injection molds needs to meet the following key requirements:High precision: Medical micro-injection molds require high precision to ensure accurate molding of tiny-sized products. The design and manufacturing of molds should take into account the size, shape and special requirements of micro-injection molded products to ensure that the mold can achieve high-precision molding.High wear resistance: Since micro-injection molded products usually require mass production, the mold needs to have high wear resistance to ensure the life and performance of the mold. Choose mold materials with good wear resistance, and use appropriate heat treatment and surface treatment techniques to improve the wear resistance of the mold.Runner system design: The design of the runner system plays an important role in the uniformity of the micro-injection molding process and the stability of product quality. The runner system should ensure that the plastic material can flow evenly and fill the mold cavity to achieve high-precision molding of micro-injection molded products.Cooling system design: The design of the cooling system is crucial to the cooling speed and product quality control of micro-injection molded products. The cooling system should be able to cool micro-injection molded products quickly and evenly to improve production efficiency and the physical properties of the product.Release system design: The design of the release system plays an important role in the smooth release of micro-injection molded products from the mold. The demoulding system should ensure that the product can escape from the mold smoothly while avoiding damage and deformation of the product.Mold processing technology: The selection and control of mold processing technology are crucial to the accuracy and quality of the mold. Commonly used mold processing techniques include CNC machining, EDM, etc. Dimensions and surface quality need to be strictly controlled during processing to ensure the accuracy and reliability of the mold.Compliance requirements: The manufacturing of medical micro-injection molds requires strict compliance with relevant regulations and standard requirements, such as ISO 13485 quality management system and FDA certification, etc. Strict quality control and inspection are required during the mold manufacturing process to ensure that the quality and performance of the mold meet the requirements.By meeting the above requirements, medical micro-injection molds can achieve high-precision, high-quality micro-injection molding product production and meet the medical industry’s demand for micro-sized products. At the same time, the mold manufacturing process needs to focus on quality control and compliance requirements to ensure the quality and safety of the mold.
- Medical clean room injection molding and TPM
TPM (Total Productive Maintenance) medical clean room can bring many benefits and is of great significance to the medical industry. Here are some of the benefits of TPM medical clean rooms:Improve product quality: Dust-free workshops can reduce the presence of dust, bacteria and other contaminants, helping to keep products clean and sterile. This reduces the risk of product contamination and cross-contamination, improving product quality and safety.Reduce the risk of contamination: Dust-free workshops reduce the generation and spread of pollutants by controlling factors such as air quality, temperature, and humidity. This helps reduce environmental pollution and employee health risks, providing a safer and healthier working environment.Improve production efficiency: Dust-free workshops improve production efficiency by reducing interference from pollutants and equipment failures. The implementation of a cleaning and maintenance plan can reduce equipment downtime and improve equipment reliability and stability.Enhance employee participation and teamwork: TPM dust-free workshop emphasizes employee participation and teamwork. Through training and awareness raising, employees can pay more attention to and value the management and maintenance of the dust-free workshop. This helps enhance employees’ sense of responsibility and teamwork, and improves overall production efficiency and quality levels.Extend the life of the equipment: The dust-free workshop extends the service life of the equipment by regularly cleaning and maintaining the equipment. This can reduce the frequency of equipment replacement and repair, and reduce equipment investment and maintenance costs.Improve brand image and market competitiveness: The implementation of dust-free workshops can enhance the brand image and market competitiveness of medical institutions or enterprises. The clean and safe environment of the dust-free workshop meets the high standards of the medical industry and helps attract the trust and choice of customers and partners.In summary, TPM medical dust-free workshops can provide a clean, safe and efficient production environment, improve product quality and production efficiency, reduce pollution risks and equipment failures, enhance employee participation and teamwork, extend equipment life, and enhance brand image and Market Competitiveness. This is of great significance to the medical industry and helps provide safer, more reliable and high-quality medical products and services. 
-
Satisfy your need for medical consumable products in AnsixTech medicalMedical consumable products are an integral part of the medical industry, including surgical instruments, dressings, catheters, syringes, needles, etc. The demand and quality control of medical consumable products are very important, involving patient safety and treatment effects.Demand: The demand for medical consumable products is closely related to the demand for medical services. As the population ages and medical technology advances, the demand for medical consumable products continues to increase. The demand for medical consumable products is also affected by the size and special needs of medical institutions.Quality control: Quality control of medical consumable products is the key to ensuring product safety and effectiveness. Quality control includes strict control from raw material procurement, manufacturing, packaging and transportation. Medical consumable products need to comply with relevant regulations and standards, such as ISO 13485 quality management system and FDA certification.Material selection: Material selection for medical consumable products is very important. Materials should be durable, non-toxic, non-irritating, and corrosion-resistant to ensure product safety and reliability. Commonly used materials include medical-grade plastics, metals, textiles, and more.Production process: The production process of medical consumable products needs to be strictly controlled to ensure product consistency and quality. The production process includes injection molding, cutting, welding, sterilization and other links, and relevant operating procedures and standards need to be strictly followed.Packaging and labeling: The packaging and labeling of medical consumable products are also important aspects of quality control. The packaging should be able to protect the product from contamination and damage, and the labeling should be clear and clear, including product name, specifications, batch number, production date and other information.Quality inspection: Medical consumable products require strict quality inspection to ensure that the products meet quality requirements. Quality inspection includes appearance inspection, dimensional measurement, functional testing, biocompatibility testing, etc. Inspection results should be recorded and traced so that quality issues can be tracked and dealt with.The demand and quality control of medical consumable products are crucial to the development of the medical industry and patient outcomes. Medical institutions and manufacturing companies should strengthen cooperation to ensure the supply and quality of medical consumable products and provide patients with safe and effective medical services.
-
Prevent ContaminationPreventing contamination is an important aspect of quality control of medical consumable products. The following are some measures to prevent contamination:Strict production environment control: The production of medical consumable products should be carried out in a clean environment, establish dust-free workshops or clean areas, control factors such as air quality, temperature and humidity, and reduce the presence of dust, bacteria and other pollutants.Strict raw material selection and supplier management: Select raw materials that meet medical industry standards and establish a qualified supplier management system to ensure the quality and safety of raw materials.Strict production process control: Establish strict production process and operating procedures to ensure the control and monitoring of each production link and reduce the generation and spread of pollutants.Strict cleaning and disinfection measures: Regularly clean and disinfect production equipment, tools and production environment to reduce the breeding and spread of bacteria and other contaminants.Strict packaging and encapsulation measures: The packaging of medical consumable products should have anti-pollution and anti-damage functions, and use standard packaging materials and encapsulation methods to ensure that the products are not contaminated during transportation and storage.Strict quality inspection and traceability system: Establish a complete quality inspection system to conduct strict inspection and testing of medical consumable products to ensure that the products meet quality requirements. At the same time, a traceability system is established to track and deal with quality issues and ensure patient safety.Training and awareness raising: Strengthen employee training and awareness raising, so that they understand and comply with relevant operating procedures and quality control requirements, and increase their attention and awareness of pollution prevention and control.Through the implementation of the above measures, we can effectively prevent the contamination of medical consumable products and ensure the quality and safety of the products. Medical institutions and manufacturing companies should strengthen cooperation and work together to ensure the supply and quality of medical consumable products and provide patients with safe and effective medical services.
-
Deliver High ToleranceInjection molding for medical services relies on uniformity. We achieve it through a streamlined process which enables us to present high tolerance. This causes little deviation in design and helps manufacture standard sizes.In order to provide high-tolerance medical consumable products, the following measures need to be taken:Choose high-tolerance materials that meet medical industry standards, such as medical-grade plastic, medical-grade silicone, etc.Conduct biocompatibility testing to evaluate the compatibility of the product with human tissue to ensure the product’s tolerance to the human body.Design appropriate size and shape to comply with human anatomy and usage habits to improve product comfort and tolerance.Make sure the product has good cleaning and disinfection properties, is easy to clean and disinfect, and can withstand commonly used cleaning and disinfectants.Conduct clinical trials and user feedback surveys to understand the tolerance and adaptability of the product in actual use, and make improvements and optimizations based on the feedback results.Establish a strict quality control system, comply with relevant regulations and standards, and ensure product compliance and tolerance.Through the implementation of the above measures, high-tolerance medical consumable products can be provided to ensure the safety and comfort of the products to the human body.
-
High Production CapabilityIn order to improve the production efficiency of medical consumable products, the following measures can be taken:Process optimization: Optimize the production process and improve production efficiency by improving the process flow, reducing processes, simplifying operations, etcAutomation equipment: Introduce automation equipment and robotic technology to replace manual operations and improve production speed and accuracy.Production line layout optimization: Optimize the layout of the production line to make the flow of materials smoother, reduce the movement time of materials and personnel, and improve production efficiency.Time-saving tools and equipment: Use time-saving tools and equipment, such as efficient injection molding machines, automated assembly equipment, etc., to reduce production cycles and labor costs.Training and skill improvement: Strengthen employee training and skill improvement, improve operating skills and production efficiency, and reduce operating errors and scrap rates.Quality control and problem solving: Establish a strict quality control system to promptly discover and solve production problems and reduce downtime and production interruptions.Carry out production planning and scheduling: Develop reasonable production plans and schedules, reasonably arrange production tasks and resources, and improve production efficiency and resource utilization.Through the implementation of the above measures, the production efficiency of medical consumable products can be improved, high-efficiency production can be achieved, and the product supply capacity and market competitiveness can be improved. At the same time, continuous improvement and optimization are required to adapt to changes in market demand and technological advancements.
-
Diverse Medical Consumable DesignsDiversified medical consumables are designed to meet different medical needs and individual patient differences, and provide better treatment effects and user experience. The following are some diverse medical consumable design directions:Size and shape: Design medical consumable products of different sizes and shapes according to the needs of different parts and usage scenarios. For example, needles, catheters, dressings, etc. can be personalized according to the body shape and condition of different patients.Material selection: Choose different materials to suit different medical needs. For example, soft materials can be used for dressings and catheters, while rigid materials can be used for surgical instruments and stents.Functions and characteristics: Design medical consumable products with different functions and characteristics according to different treatment purposes and patient needs. For example, adjustable catheters, self-adhesive dressings, reusable instruments, etc.Humanized design: Considering the comfort and convenience of patients, design humanized medical consumable products. For example, easy-to-operate syringes, comfortable dressings, easy-to-clean instruments, etc.Sustainable design: Considering environmental protection and sustainable development, design degradable and recyclable medical consumable products to reduce negative impact on the environment.User participation: Collaborate and provide feedback with medical professionals and patients to understand their needs and opinions and incorporate them into the design process to ensure the practicality and adaptability of medical consumable products.Through diversified medical consumable designs, we can provide better treatment effects, improve patient comfort and convenience, and meet different medical needs and individual differences. At the same time, quality control and compliance requirements also need to be considered to ensure product safety and reliability.The functioning and applicability of medical consumables and medical disposables are the foundations of our designs. For mass manufacturing, we may adjust the sizes and give a wide size range as per the client’s needs.
-
High Quality Medical ConsumableProducing high-quality medical consumables requires following strict quality control and compliance requirements. The following are general steps for producing high-quality medical consumables:Design and development: Design and develop medical consumable products according to market demand and medical industry standards. This includes determining the product’s functionality, dimensions, material selection, etc.Material procurement: Choose high-quality materials that meet medical industry standards and establish cooperative relationships with reliable suppliers. Ensure material quality and traceability.Production process planning: Develop production process flow and operating procedures to ensure product consistency and quality. Factors such as production efficiency, process control and quality inspection are taken into consideration.Preparation of production equipment and tools: Prepare appropriate production equipment and tools to ensure that they meet production requirements and quality control standards. Equipment should be regularly maintained and calibrated to ensure proper operation and accuracy.Production process control: During the production process, all links are strictly controlled, including raw material ingredients, injection molding, assembly, cleaning and disinfection, etc. Ensure that every link meets quality requirements and operating procedures.Quality inspection and testing: Conduct strict quality inspection and testing on the produced medical consumable products, including appearance inspection, dimensional measurement, functional testing, biocompatibility testing, etc. Ensure products meet quality standards and compliance requirements.Packaging and labeling: Properly package and label products to ensure that they are not contaminated and damaged during transportation and storage. Packaging should comply with relevant regulations and standards.Quality control and continuous improvement: Establish a strict quality control system, including quality records, traceability and problem solving. Continuously improve the production process and product quality to adapt to market demand and technological progress.Through the implementation of the above steps, high-quality medical consumable products can be produced to ensure product safety, reliability and effectiveness. Medical institutions and manufacturing companies should strengthen cooperation and work together to ensure the supply and quality of medical consumable products and provide patients with high-quality medical services.
-
Micro Medical Disposables ManufacturingMicro medical disposables are manufactured with size requirements that go into microns. Our micro products fall in the range of 0.1g to 0.5g, as the team caters to the top medical disposable companies.The standards for disposable micro medical supplies are an important basis for ensuring product quality and safety. Here are some common standards for disposable micro medical supplies:ISO 13485: This is an international standard for medical device quality management systems. It stipulates the quality management requirements that medical device manufacturers should follow, including design and development, production, sales and service.ISO 10993: This is an international standard for the biocompatibility of medical devices. It stipulates the biocompatibility requirements for medical devices when they come into contact with human tissue, including testing requirements for cytotoxicity, skin irritation, and allergenicity.ASTM F2100: This is the standard for disposable masks, which stipulates the material, design, performance and testing requirements of masks, including bacterial filtration efficiency, breathing resistance, liquid resistance and other indicators.EN 455: This is the standard for disposable medical gloves, which specifies the material, size, performance and testing requirements of gloves, including tensile strength, puncture resistance, water leakage test and other indicators.EN 13795: This is a standard for disposable surgical gowns and drapes. It specifies the material, design, performance and testing requirements for surgical gowns and drapes, including resistance to liquid permeability, anti-bacterial permeability and other indicators.FDA 510(k): This is the U.S. Food and Drug Administration’s (FDA) medical device premarket approval requirement, which stipulates the safety and effectiveness evaluation requirements for disposable micro medical supplies.The above standards are common standards for disposable micro medical supplies. Manufacturers should select the applicable standards based on the characteristics and uses of the products and ensure that the products meet the requirements of the standards. At the same time, you should also comply with the regulations and legal requirements of relevant countries and regions to ensure product compliance and safety.

- Over 25+ Yeasrs Experience of Manufacturing China Medical DisposablesAnsixTech Medical has been in the field of manufacturing medical consumables and disposables for more 。More than 25 years of experience in manufacturing medical disposables in China has allowed us to observe some trends and development directions:Technological innovation: With the continuous advancement of science and technology, the manufacturing technology of medical disposable products is also constantly innovating. For example, advanced material science, nanotechnology and 3D printing are introduced to improve product performance, durability and safety.Automated production: The application of automated production technology is becoming more and more widespread, which can improve production efficiency, reduce costs, and ensure product consistency and quality. The introduction of automated production lines and robotics will become a future trend.Environmentally friendly and sustainable: The awareness of environmental protection and sustainable development continues to increase, and the manufacturing of medical disposables is increasingly focusing on environmental friendliness. For example, using degradable materials, reducing waste generation and energy consumption, etc., to reduce negative impact on the environment.Personalized customization: With the development of personalized medical treatment, medical disposables also tend to be personalized. Based on the specific needs of patients and the recommendations of medical professionals, customized products will better meet the needs of different patientsDataization and intelligence: The manufacturing of medical disposables will be increasingly integrated with data and intelligent technology. Through data analysis and intelligent monitoring, the optimization of the production process and the improvement of quality control can be achieved.International cooperation and market expansion: Chinese medical disposable products manufacturers will continue to strengthen cooperation with international partners and improve the international competitiveness of their products. At the same time, we actively explore international markets to meet global medical needs.These trends will promote the development of China’s medical disposables manufacturing industry and improve product quality and innovation capabilities. Manufacturers need to constantly pay attention to market demand and technological progress, and actively adapt to changes to meet the needs of the medical industry and provide better medical services to patients.
-
How to choose raw materials for medical consumablesSelecting the right material for injection molding is important because the tolerance, performance, and strength depend on it. We work with medical-grade materials and operate as bulk medical supplies.Selecting raw materials for medical consumables is an important step to ensure product quality and safety. Here are some guidelines to help select raw materials for medical consumables:Comply with medical industry standards: Choose raw materials that comply with medical industry standards, such as ISO standards, FDA certification, etc. These standards ensure that raw materials meet quality and safety requirements.Biocompatibility: Consider the biocompatibility of raw materials, that is, whether they will cause adverse reactions when in contact with human tissue. Choose materials with good biocompatibility, such as medical-grade plastic, medical-grade silicone, etc.Physical properties: Select raw materials with appropriate physical properties based on the product’s use and performance requirements. For example, corrosion resistance, high temperature resistance, flexibility, strength, etc.Processability: Consider the processability of raw materials, that is, whether they can be easily processed into the required shape and size. Choose raw materials that are easy to process to facilitate shaping, cutting, welding and other operations during the production processTraceability: Ensure that raw materials are traceable, that is, their origin and production process can be traced. This helps ensure the quality and compliance of raw materials.Sustainability: Consider the sustainability of raw materials, i.e. environmental impact and recyclability. Choose degradable and recyclable raw materials to reduce negative impact on the environment.Compliance requirements: Comply with relevant regulations and standards, such as REACH, RoHS, etc. Ensure that raw materials meet the restrictions and requirements of regulations and standards.When selecting raw materials for medical consumables, manufacturers should comprehensively consider the product’s purpose, performance requirements, biocompatibility, processability, traceability, sustainability and compliance requirements. At the same time, we establish cooperative relationships with reliable suppliers to ensure the quality and reliability of raw materials.
- Silicone injection molding
Silicone injection molding works extremely well as it provides both chemical and electrical resistance. Silicone fares well at room temperature without losing its strength. Many hospital consumables suppliers offer silicone as the base material.Silicone is a material commonly used in the manufacture of medical consumables and has the following properties:Biocompatibility: Silicone has good biocompatibility and will not cause obvious toxicity or irritation when in contact with human tissue. This makes silicone ideal for manufacturing medical consumables such as catheters, dressings and artificial organs.Softness: Silicone has good softness and elasticity and can adapt to different parts and curve shapes. This allows the silicone product to fit comfortably against the patient’s body, reducing discomfort.High temperature resistance: Silicone has high temperature resistance and can maintain stability and performance under high temperature conditions. This allows silicone products to be used in high-temperature sterilization and disinfection processesCorrosion resistance: Silicone has good corrosion resistance and can resist the erosion of chemical substances such as acids and alkalis, solvents and oxidants. This allows silicone products to maintain stability and reliability in various environments.Transparency: Silicone has good transparency and can transmit light, allowing silicone products to provide clear vision during medical operations and observation.Wear resistance: Silicone has high wear resistance and can resist friction and wear. This gives silicone products a long life and durability.Processability: Silicone has good processability and can be used to manufacture products of various shapes and sizes through injection molding, extrusion molding, calendering and other processes.In general, silicone has good biocompatibility, softness, high temperature resistance, corrosion resistance, transparency, wear resistance and processability, making it one of the commonly used materials in the manufacturing of medical consumables. . 

- Medical rubber moldingRubber precision equipment is great, especially when the client's favor high tolerance production. There is a higher demand for high precision equipment when it comes to rubber medical consumables, especially with regard to micro injection molding.Medical rubber molding refers to the process of manufacturing rubber materials into medical consumable products through a molding process. The following are the general steps for medical rubber molding:Raw material preparation: Choose medical rubber materials that meet the standards of the medical industry, such as nitrile rubber (NBR), styrene-butadiene rubber (BR), silicone, etc. Ensure the quality and traceability of raw materials.Rubber preparation: Mix the rubber material with additives (such as vulcanizing agents, processing aids, etc.), and prepare the rubber through a rubber mixer or rubber extruder to make the rubber material have appropriate fluidity and processability.Molding process selection: According to the shape and size requirements of the product, select the appropriate molding process, such as injection molding, extrusion molding, calendar molding, etc. Different processes have different application scopes and characteristics.Mold manufacturing: According to the design requirements of the product, the corresponding molds are manufactured. The mold can be a metal mold, a silicone mold, or a 3D printing mold, etc., used to give the rubber material the desired shape.Molding process: The rubber material is heated to the appropriate temperature, and then the rubber material is injected or extruded into the mold through equipment such as an injection molding machine, extruder or calender. In the mold, the rubber material is cured and shaped to the shape and size of the mold.Post-processing: Molded rubber products require post-processing, such as trimming, cleaning, and removal of mold residues. Ensure product appearance and quality.Quality control and inspection: Quality control and inspection of molded rubber products, including appearance inspection, dimensional measurement, physical performance testing, etc. Ensure products meet quality standards and compliance requirements.Through the implementation of the above steps, medical rubber materials can be manufactured into medical consumable products of various shapes and sizes. Manufacturers need to select appropriate rubber materials and molding processes based on product requirements and market demand to ensure product quality and safety.
- Polypropylene (PP) Molding
A propylene mold is excellent when you require heat insulation. Apart from that, there is incredible flexibility that you are less likely to use from any other plastic material. The strength is also comparable to that of other robust materials.Polypropylene (PP) is a commonly used plastic material that is widely used in the manufacturing of medical consumables. The following are the general steps for polypropylene molding:Raw material preparation: Select polypropylene raw materials that meet medical industry standards to ensure the quality and traceability of raw materials.Melting: Add the polypropylene raw material into the hopper of the injection molding machine, and convert the polypropylene raw material into a molten state that can be injection molded through heating and melting.Injection molding: Molten polypropylene is injected into the mold of an injection molding machine. The mold can be a metal mold, manufactured according to the design requirements of the product. The injection molding machine fills the molten polypropylene into the cavity of the mold through pressure and temperature control, and solidifies it after a certain cooling time.Cooling and demoulding: After injection molding, the polypropylene in the mold needs to be cooled to solidify and stabilize it. Cooling time depends on the size and thickness of the product. After cooling is completed, the mold is opened and the formed polypropylene product is taken out of the mold.Post-processing: Molded polypropylene products require post-processing, such as trimming, cleaning, and removal of mold residues. Ensure product appearance and quality.Quality control and inspection: Quality control and inspection of molded polypropylene products, including appearance inspection, dimensional measurement, physical performance testing, etc. Ensure products meet quality standards and compliance requirements.Through the implementation of the above steps, polypropylene can be manufactured into medical consumable products of various shapes and sizes. Manufacturers need to select appropriate polypropylene raw materials and injection molding processes based on product requirements and market demand to ensure product quality and safety. 

- Teflon Injection Molding for Medical PFAWe offer various surface finish options for custom plastic injection molder parts. Some of the common finishing choices are PM-F0, PM-F1, PM-F2, SPI-C1, PM-T1, PM-T2, SPI-B1, SPI-A2, etc. In addition, we also offer Industry standard Mold-Tech finish choices. We use mold mirror EDM treatment, mold surface polishing mirror, mold surface electroplating mirror treatment, mold surface PVD treatment, etc.
- TPU material injection moldingThermoplastic Polyurethane (TPU for short) is a unique elastomer material with the following characteristics and advantages:Elasticity and wear resistance: TPU has excellent elasticity and wear resistance, and can maintain its elasticity and durability under long-term use and high stress conditions. This makes TPU suitable for applications that require a high degree of elasticity and wear resistance, such as shoe soles, seals, etc.Oil resistance and chemical resistance: TPU has good oil resistance and chemical resistance, and can resist the erosion of oil, solvents and chemicals. This makes TPU advantageous in applications involving oil and chemical contact, such as pipes, seals, etc.High temperature resistance: TPU has high temperature resistance and can maintain stability and performance in high temperature environments. This makes TPU advantageous in applications that require high temperature resistance, such as automotive parts, electronic equipment, etc.Processability: TPU has good processability and can be manufactured into products of various shapes and sizes through injection molding, extrusion molding, calendering and other processes. TPU materials can be combined with other materials (such as textiles, metals, etc.) to meet the needs of different applications.Biocompatibility: TPU material has good biocompatibility and will not cause obvious toxicity or irritation when in contact with human tissue. This makes TPU potential in applications in the medical field, such as medical devices, medical catheters, etc.Recyclability: TPU materials are recyclable and can be reprocessed and reused to reduce negative impact on the environment.In general, TPU has excellent elasticity, wear resistance, oil resistance, chemical resistance, high temperature resistance, processability, biocompatibility and recyclability, making it a widely used material in various fields. one of the materials.

-
Why AnsixTech medical as Hospital Medical Consumables & Disposables ManufacturerWhen it comes to plastics used in medical devices, professional medical disposables manufacturers are the best bet for any kind of order. Whether it is product design and development, technical team, industry work experience, supply chain integration capabilities, mold design, mold flow analysis,assembly, molding, quality assurance, logistics and transportation or hygiene, our team members are trained enough to handle it all.Rich experience and expertise: AnsixTech Medical has more than 25 years of manufacturing experience and has in-depth understanding and professional knowledge of medical consumables and disposables manufacturing. They understand the needs and standards of the medical industry and are able to provide products that meet quality and safety requirements.High-quality products: AnsixTech Medical is committed to providing high-quality medical consumables and disposables. They select high-quality raw materials, adopt advanced production processes and strict quality control to ensure product consistency and reliability.Complete quality management system: AnsixTech Medical has established a complete quality management system, including quality control, quality inspection and quality records. They focus on quality control in every link to ensure that products meet quality standards and compliance requirements.Innovation and technological progress: AnsixTech Medical continuously pursues innovation and technological progress, introducing advanced material science, production processes and equipment. They work with scientific research institutions and partners to continuously improve product performance and functionality to meet changing market needs.Customer customization and service: AnsixTech Medical can provide customized products and solutions according to customer needs. They establish good cooperative relationships with customers and provide timely technical support and after-sales service.Compliance and sustainable development: AnsixTech Medical focuses on product compliance and sustainable development. They comply with relevant regulations and standard requirements, use environmentally friendly and recyclable materials, and reduce negative impacts on the environment.By choosing AnsixTech Medical as a manufacturer of hospital medical consumables and disposables, you can get high-quality products, professional knowledge and services to meet the needs of the medical industry and provide patients with high-quality medical services.
-
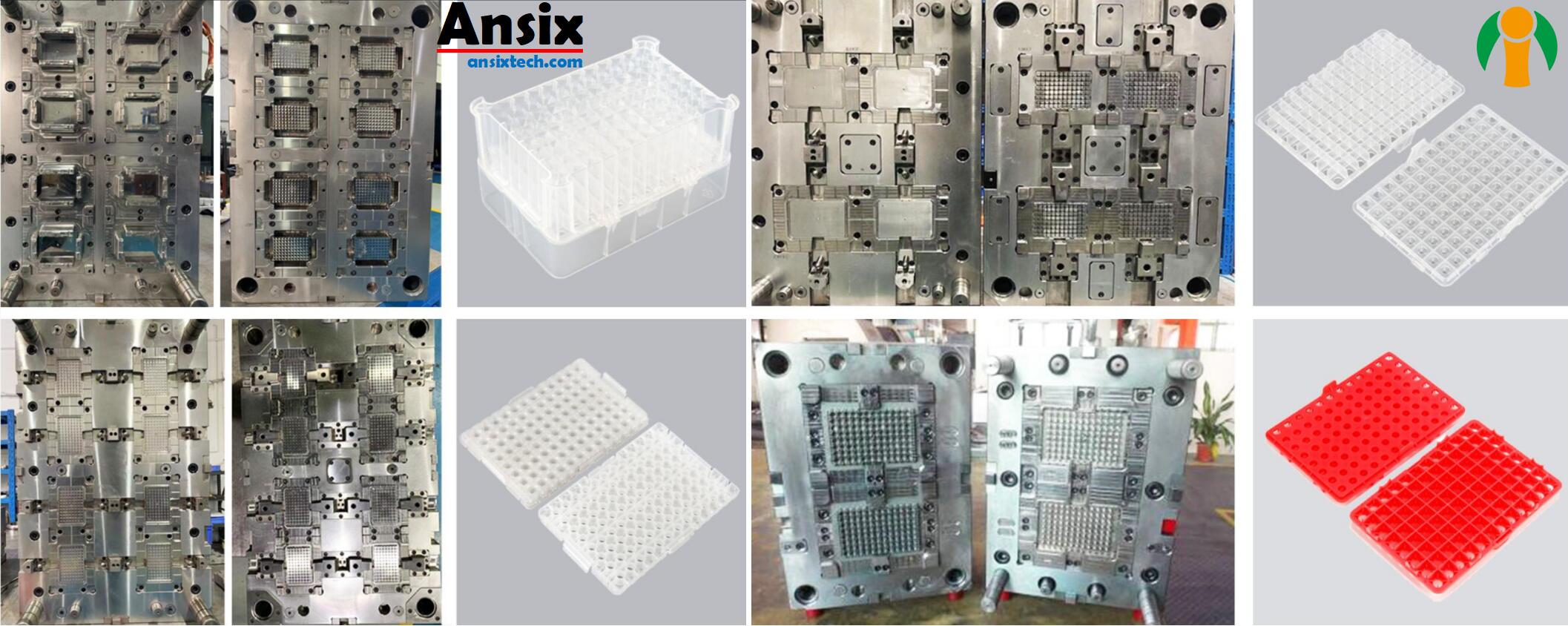

- AnsixTech Medical Related CasesAnsixTech Medical, as a manufacturer of medical consumables and disposables with extensive experience, may have cooperated with many hospitals and medical institutions and provided various products and solutions. You can get more information about the cases they work with customers by visiting AnsixTech Medical’s official website, reading their customer cases, or contacting them directly. This will help you better understand their products and services and assess whether they fit your needs.Check through our cases and see how we solve problems with robust injection molding capabilities.
AnsixTech Medical Related Cases
-
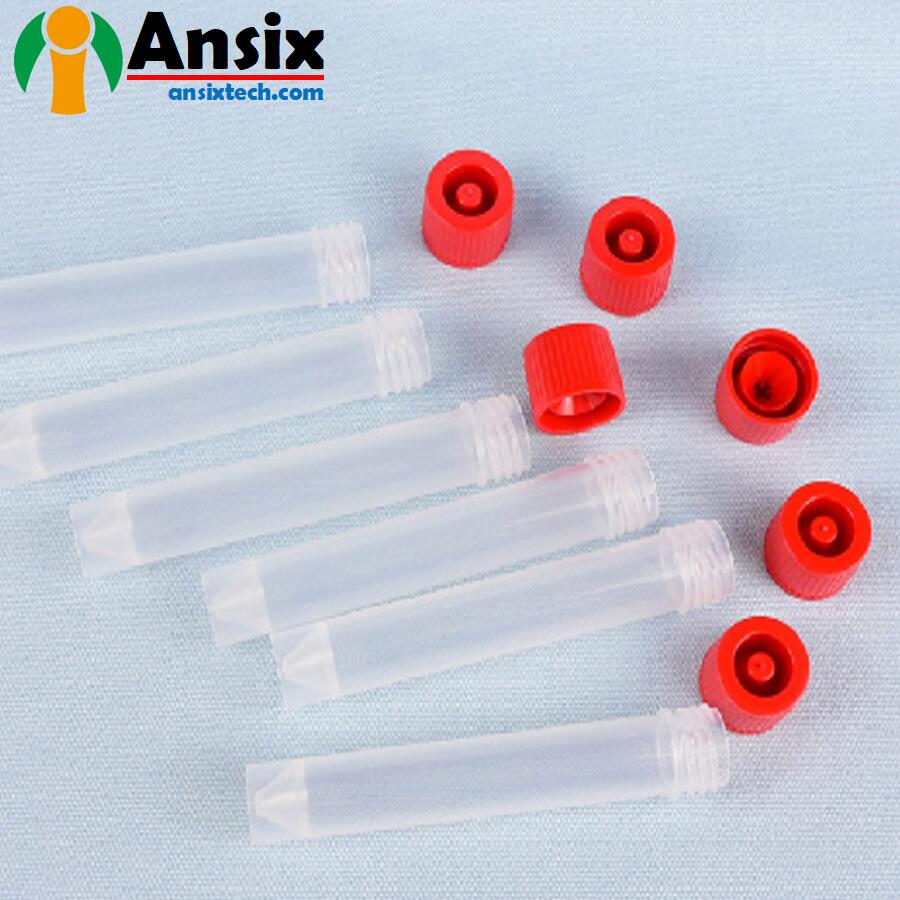 Sampling Tube 19
Sampling Tube 19 -
 Ruhr Joint 20
Ruhr Joint 20 -
 Petri Dish 21
Petri Dish 21
contact usTry Our Problem-solving Injection Molding Services Now
AnsixTech Medical serves you with medical injection molding solutions from design to tooling to material selection and manufacturing. Contact our specialized team and solve your problem now.
ISO 13485 Certified factory
ISO 8 Cleanroom
Robust Expertise for Medical Injection Molding
Advanced mold manufacturing capabilities and plastic injection molding equipment
Quick Response within 12 hours
If you have any questions about products in the medical field, please send us a message(Email: info@ansixtech.com ) at any time and our team will reply to you within 12 hours.