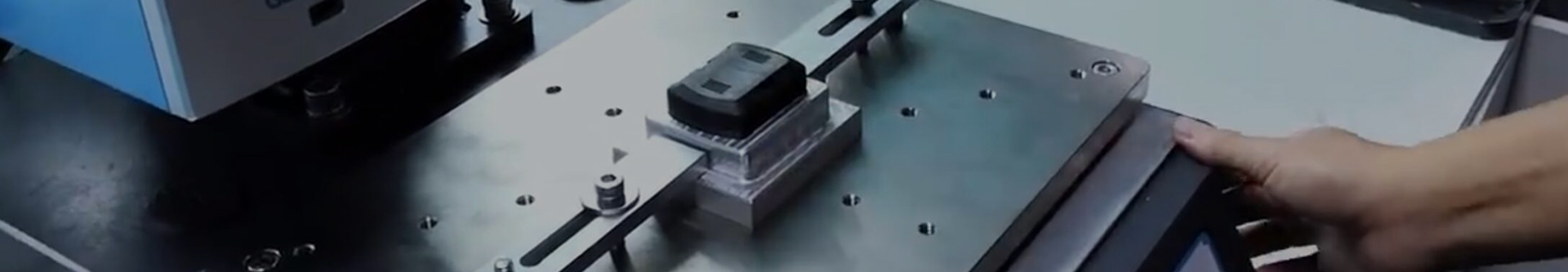
Laryngoscope Guard medical plastic injection mold
FEATURES

- Laryngoscope Guard medical plastic injection mold
The application of medical plastic injection molds for laryngoscope protective covers is mainly used to produce laryngoscope protective cover products. A laryngoscope shield is a medical device used to protect medical staff and patients, usually made of medical-grade plastic material. Its main function is to provide a protective barrier to reduce the risk of droplet transmission and cross-infection during laryngoscopy or laryngoscopy surgery.The application of medical plastic injection molds for laryngoscope protective covers is mainly reflected in the following aspects:Production of laryngoscope protective cover products: By using laryngoscope protective cover medical plastic injection molds, large-scale production can be carried out to manufacture laryngoscope protective cover products that meet design requirements and quality standards.
Product Materials:
Medical PC
Mold Material:
S136H
Number of Cavities:
1*1
Glue Feeding Method:
Cold runner
Cooling Method:
Water cooling
Molding Cycle
32.5s
- The process of disposable laryngoscope shield mold design
Disposable laryngoscope shield mold DFM (Design for Manufacturability) is an important step before mold design, aiming to evaluate and confirm the manufacturability of the designMaterial selection: Confirm the availability and suitability of the selected materials, including single-use medical-grade plastic materials medical PCStructural design: Evaluate the structural design of the mold, including the shape, size, wall thickness, etc. of the protective cover to ensure that the mold can meet the requirements of the product and achieve good filling and cooling effects during the injection molding process.Mold separation: Confirm whether the separation and demoulding of parts need to be considered in the mold design to ensure that the mold can smoothly remove the injection molded parts from the mold to avoid damage or deformation.Injection molding process: Evaluate injection molding process parameters, such as injection pressure, injection speed, cooling time, etc., to ensure that the mold design can adapt to the required injection molding process conditions.Mold manufacturing: Consider the feasibility and cost-effectiveness of mold manufacturing, including the difficulty of mold processing, process flow, manufacturing cycle, etc., to ensure that the mold can be manufactured within a reasonable time.By conducting DFM of disposable laryngoscope shield molds, potential manufacturing problems can be discovered and solved early, the manufacturability and production efficiency of the mold can be improved, and later modification and adjustment costs can be reduced. ensure the quality and feasibility of the mold design. 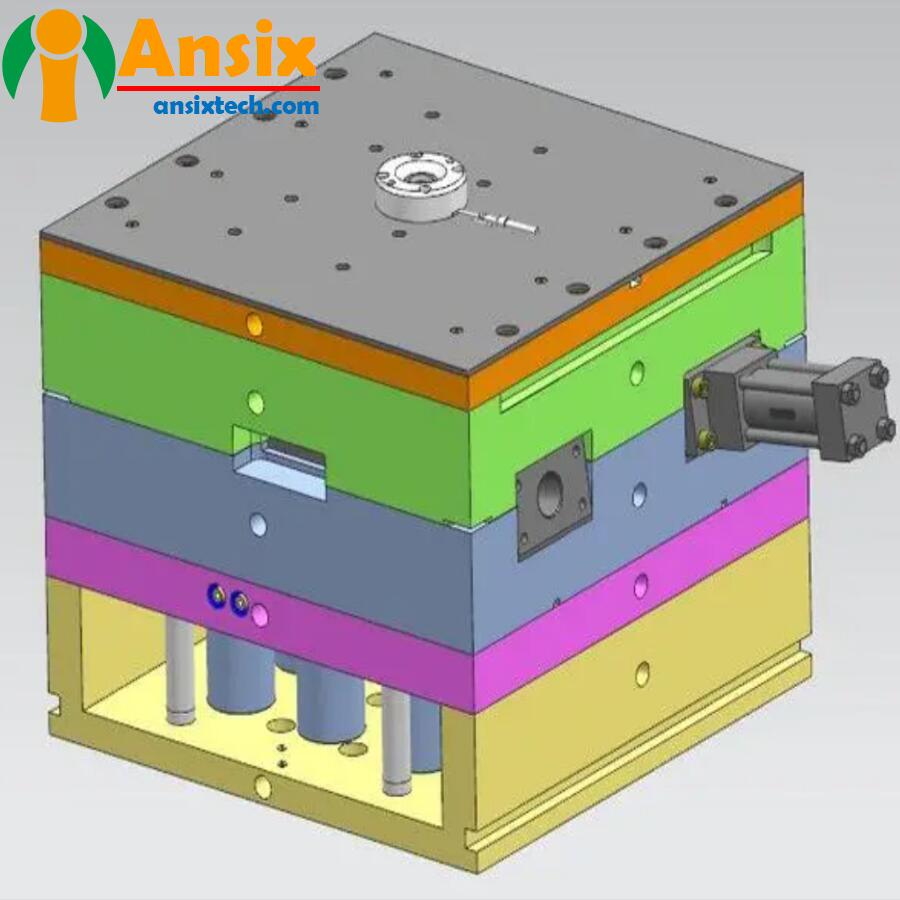
-
The mold 3D design:Determine the design requirements: Communicate with the customer or product development team to understand the design requirements of the disposable laryngoscope protective cover, including size, shape, material, etc.Concept design: According to the design requirements, carry out preliminary conceptual design, including the overall structure of the mold, clamping method, separation method, etc.3D model design: Use computer-aided design (CAD) software to convert conceptual designs into specific three-dimensional models. During the design process, factors such as the size, wall thickness, and clamping port shape of the mold are taken into consideration.Mold detailed design: Based on the 3D model, carry out detailed mold design, including mold parts, assembly methods, cooling system, exhaust system, etc.Mold analysis: Use mold flow analysis software to conduct flow analysis on the mold, evaluate the filling situation and cooling effect during the injection molding process, and optimize the mold design.Mold manufacturing: According to the mold design, the mold is manufactured. This includes the selection of mold materials, determination of processing technology, processing and assembly of parts, etc.Mold debugging: Install the mold on the injection molding machine to debug and test the mold. Adjust the injection molding process parameters and check the clamping effect of the mold and the quality of the injection molded parts.Sample verification: Carry out trial mold production and produce samples of disposable laryngoscope protective covers. Inspect and verify samples to ensure they meet design requirements and quality standards.Mass production: After sample verification, mass production of disposable laryngoscope protective covers will be carried out. Depending on the needs, you can choose single-cavity molds or multi-cavity molds for production.Throughout the design process, close communication and collaboration with customers and relevant teams are required to ensure that the mold design meets needs and standards. At the same time, it is also necessary to comply with relevant quality management systems and regulatory requirements to ensure that mold manufacturing complies with relevant standards and regulations for medical devices.

- The manufacturing and assembly of disposable laryngoscope shield molds:
Mold parts processing: According to the mold design, each part of the mold is processed. This includes milling, turning, drilling, wire cutting and other processes, as well as heat treatment of mold parts.Mold parts assembly: Assemble the processed mold parts. This includes assembling the various parts of the mold according to the design requirements, such as the mold base plate, mold core, mold cavity, etc.Mold surface treatment: Surface treatment of the mold, such as grinding, polishing, electroplating, etc., to improve the surface finish and wear resistance of the mold.Mold debugging: Install the assembled mold on the injection molding machine to debug and test the mold. This includes adjusting injection molding process parameters, checking the clamping effect of the mold and the quality of the injection molded parts.Sample verification: Carry out trial mold production and produce samples of disposable laryngoscope protective covers. Inspect and verify samples to ensure they meet design requirements and quality standards.Mass production: After sample verification, mass production of disposable laryngoscope protective covers will be carried out. Depending on the needs, you can choose single-cavity molds or multi-cavity molds for production.During the entire manufacturing and assembly process, the dimensional accuracy, surface quality and assembly accuracy of the mold need to be strictly controlled to ensure that the disposable laryngoscope shield manufactured by the mold has good quality and performance. At the same time, it is also necessary to comply with relevant quality management systems and regulatory requirements to ensure that mold manufacturing complies with relevant standards and regulations for medical devices.
- When choosing the plastic material for a disposable laryngoscope
When choosing the plastic material for a disposable laryngoscope shield, there are several factors to consider:Medical grade: Ensure that the selected plastic material complies with relevant standards and regulations for medical devices and has medical grade safety and durability.Transparency: Disposable laryngoscope protective covers need to have good transparency to ensure that medical staff can clearly observe the patient’s larynx.Chemical resistance: Choose plastic materials with good chemical resistance that can resist corrosion from common disinfectants and cleaning agents and maintain the performance and appearance of the laryngoscope shield.Wear resistance: Consider choosing a plastic material with good wear resistance to ensure that the laryngoscope shield is not easily scratched or worn during use.Flexibility: Choose a plastic material with a certain degree of flexibility that can adapt to the throat shape of different patients and provide a comfortable use experience.Commonly used medical grade plastic materials include polypropylene (PP), polyethylene (PE), polycarbonate (PC), etc. This time the customer chose to use medical grade PC to produce this project.These materials have good transparency, chemical resistance, and abrasion resistance, making them suitable for use in the manufacture of disposable laryngoscope shields.Cost factors and availability also need to be considered when choosing plastic materials. It is best to work with plastic suppliers and customer to select the appropriate plastic material based on specific needs and ensure that it complies with relevant standards and regulations for medical devices. 

- Disposable laryngoscope shield mold manufacturing validation
Disposable laryngoscope shield mold manufacturing validation is an important step to ensure mold manufacturing quality and performance.Sample inspection: Produce samples of disposable laryngoscope protective covers and conduct inspection. Check whether the size, shape, surface quality, etc. of the sample meet the design requirements and quality standards.Functional test: Functionally test the samples to simulate actual usage conditions to ensure that the disposable laryngoscope protective cover can be used normally and provide effective protection.Injection molding process verification: By adjusting injection molding process parameters, such as injection pressure, injection speed, cooling time, etc., verify the filling status and cooling effect of the mold during the injection molding process.Mass production verification: Before mass production, conduct small batch production verification. Produce a certain number of disposable laryngoscope shields and check the consistency and stability of the product.Quality control: Establish a quality control system, including inspection and testing standards, process control, records and traceability, etc., to ensure that mold manufacturing complies with relevant standards and regulations for medical devices.Customer acceptance: Submit the produced disposable laryngoscope protective cover samples to the customer for acceptance to ensure that the product meets the customer’s requirements and expectations.Through the above verification methods, it can be ensured that the quality and performance of disposable laryngoscope shield mold manufacturing meet the design requirements and quality standards. It is best to have mold manufacturing verification performed by an experienced mold maker to ensure the quality and reliability of the mold. At the same time, maintain close communication and collaboration with customers and relevant teams to solve problems in a timely manner to ensure the smooth progress of manufacturing verification.
- Medical Plastic Injection Molding Capabilities
AnsixTech has four production bases in China and Vietnam. We have a total of 260 injection molding machines. The tonnage of injection molding machines ranges from the smallest 30 tons to 2800 tons. The main injection molding machines include Japan's Fanuc, Sumitomo, Toshiba, Nissei, and Germany's Arburg (mainly liquid silicone injection molding, mainly two component). China has Haitian and Victor Taichung Machinery, etc.AnsixTech medical injection molding experts are a strong professional team with extensive knowledge and experience in the field of medical injection molding.Technical expertise: AnsixTech medical injection molding experts have extensive technical expertise and are familiar with all aspects of medical injection molding. our understand the special requirements of medical injection molding, including material selection, design optimization, mold manufacturing, injection molding process, quality control, etc. our maintain knowledge of the latest technologies and trends in the industry through continuous learning and research.Design capabilities: AnsixTech medical injection molding experts have excellent design capabilities. our have a professional design team that can carry out mold design and product design optimization according to customer needs and product requirements. our are familiar with CAD/CAM software and tools and are able to perform precise mold design and product structure optimization to improve the efficiency and quality of injection molding.Mold manufacturing: AnsixTech medical injection molding experts have advanced mold manufacturing equipment and processes. Our use advanced processing technology and processes to manufacture high-quality medical injection molds. Our pay attention to details and precision control to ensure the accuracy and stability of the mold. our also work with excellent mold manufacturers to ensure the quality and delivery time of the molds.Injection molding production: AnsixTech medical injection molding experts have advanced injection molding equipment and processes, enabling efficient and stable injection molding production. our master the process requirements of medical injection molding and can perform injection molding production according to customer needs to ensure product quality and consistency. our focus on the control and optimization of process parameters to improve production efficiency and product quality.Quality Control: AnsixTech medical injection molding experts attach great importance to quality control. our understand the quality standards and regulatory requirements for medical injection molded products and can help customers establish and implement strict quality control systems. our use advanced testing equipment and methods to conduct product quality inspection and testing to ensure that products comply with relevant standards and regulations.Project management: AnsixTech medical injection molding experts have excellent project management capabilities. our are able to work closely with clients to understand their needs and requirements and develop detailed project plans and timelines. our focus on communication and coordination to ensure projects are delivered on time and maintain good working relationships with clients.Innovation ability: AnsixTech medical injection molding experts focus on innovation and technological progress. our constantly explore new materials, processes and technologies to provide better quality medical injection molding solutions. our actively participate in industry seminars and exhibitions, communicate and share experiences with experts in the same industry, and continuously improve their innovation capabilities and competitiveness.AnsixTech medical injection molding experts are able to provide customers with high-quality, efficient and reliable medical injection molding solutions due to their technical expertise, design capabilities, mold manufacturing, injection molding production, quality control, project management and innovation capabilities. Our are committed to working with customers to jointly promote the development and progress of the medical injection molding industry. 
- AnsixTechMedical Plastic Injection Molding Quality Assurance
AnsixTech factories have strict quality assurance measures in medical plastic injection molding to ensure product quality and safety. The following are the AnsixTech factory’s measures for quality assurance in medical plastic injection molding:Material selection: AnsixTech factories use high-quality medical-grade plastic materials, which comply with the standards and requirements of the international medical industry. The factory will conduct strict testing and screening of raw materials to ensure the quality and reliability of the materials.Process control: The factory has an experienced team of process engineers who will strictly control and optimize the injection molding process parameters. The factory will use advanced injection molding equipment and technology to ensure the dimensional accuracy, surface quality and structural integrity of the product.Quality inspection: AnsixTech factory has complete quality inspection equipment and processes. The factory will conduct comprehensive quality inspections on the injection molding process, including dimensional measurement, appearance inspection, physical performance testing, etc. The factory also conducts batch sampling inspections to ensure product consistency and reliability.Quality management system: We have the international medical certification ISO13485 system, and have obtained an ISO 8 Cleanroom, which complies with the US medical grade FDA510K standard, and strictly implements it in accordance with the standards. The factory conducts internal audits and external certifications to ensure the effectiveness and continuous improvement of the quality management system.Compliance: AnsixTech factories follow relevant regulations and standards, such as FDA requirements and other international medical industry standards. The factory will ensure product compliance, including the biocompatibility of materials, product safety and reliability, etc.AnsixTech factories have strict quality assurance measures in medical plastic injection molding to ensure product quality, safety and compliance. The factory is committed to providing high-quality medical plastic injection molding products to meet the needs of customers and the market.
- Why Choose AnsixTech Medical Plastic Injection Services?Professional experience: AnsixTech has many years of experience in medical plastic injection services and is very familiar with the needs and standards of the medical industry. our are able to provide high-quality injection services and ensure product safety and reliability.Advanced equipment: AnsixTech has advanced injection equipment and technology, which can meet various complex injection needs. our constantly update their equipment and technology to ensure they can provide the latest solutions.Quality control: We have the international medical certification ISO13485 system, and have obtained an ISO 8 Cleanroom, which complies with the US medical grade FDA510K standard, and strictly implements it in accordance with the standards.AnsixTech focuses on quality control, and they adopt a strict quality management system to ensure that each product meets relevant standards and requirements. our conduct rigorous inspection and testing to ensure product quality and performance.AnsixTech can effectively control costs and improve the company’s competitiveness and profitability. our focus on supply chain management, production process optimization, energy and resource conservation, overhead control and quality management to reduce costs in many aspects.Customer customization: AnsixTech can provide customized injection services according to customer needs. our work closely with customers to understand their needs and requirements and provide solutions accordingly.Quality service: AnsixTech focuses on customer service, they provide timely and professional technical support to ensure customer satisfaction. our are able to respond quickly to customer needs and provide solutions.To sum up, choosing AnsixTech medical plastic injection services can provide you with professional experience, advanced equipment, high-quality services and customized solutions to ensure product quality and performance.Cost control:1.Supply chain management: AnsixTech establishes long-term cooperative relationships with suppliers and conducts effective supply chain management. our negotiate with suppliers for better prices and preferential terms, thereby reducing the purchase cost of raw materials and equipment. In addition, our regularly evaluate supplier performance to ensure the stability and efficiency of the supply chain.2.Production process optimization: AnsixTech improves production efficiency and reduces production costs by optimizing the production process. our reduce labor and time costs through refined management and automated equipment. our also regularly evaluate and improve production processes to increase efficiency and reduce costs.3.Save energy and resources: AnsixTech focuses on saving energy and resource use, and reduces the waste of energy and resources through the use of efficient equipment and processes. our have also adopted recycling and reuse measures to reduce the cost of waste disposal. In addition, our regularly evaluate and improve energy and resource usage to further reduce costs.4.Management expense control: AnsixTech reduces management expenses through refined management and control of various expenses. They conduct strict budgeting and monitoring of various expenses to ensure the rationality and effectiveness of expenses. our also regularly evaluate and optimize overhead expenses to further reduce costs.5.Quality management: AnsixTech focuses on quality management and reduces the cost of after-sales service and returns by improving product quality and reducing the rate of defective products. our conduct strict quality control and testing to ensure that products meet standards and requirements. our also regularly evaluate and improve quality management to further reduce costs. If you have any questions about products in the medical field, please send us a message(Email: info@ansixtech.com ) at any time and our team will reply to you within 12 hours.
- AnsixTech Custom Medical Device Injection Molding ProcessDemand analysis: AnsixTech communicates with customers to understand their needs and requirements. our discuss the details of medical device functionality, design requirements, material selection, etc. with customers to ensure an accurate understanding of customer needs.Design and mold making: AnsixTech’s engineering team designs and molds medical devices based on customer needs and requirements. our use CAD and other design software for 3D modeling and mold design of products. During the design process, our take into account factors such as the structure, function, and injection molding process of the medical device.Material selection: AnsixTech selects appropriate medical-grade plastic materials based on the requirements of the medical device and the use environment. our consider the material’s high temperature resistance, corrosion resistance, biocompatibility and other properties to ensure the safety and reliability of the material.Injection molding process: AnsixTech formulates an injection molding process plan based on the design and mold production of medical devices. our take into account parameters such as injection temperature, injection pressure, and injection speed to ensure that the injection molding process of medical devices is stable and the quality is controllable.Quality control: AnsixTech conducts strict quality control throughout the entire injection molding process. our monitor and inspect the injection molding process to ensure that the dimensional accuracy, appearance quality and functional performance of the product meet the requirements. our also conduct product reliability testing and verification to ensure product safety and reliability.AnsixTech can provide customized medical device injection molding process services. our work closely with customers to conduct demand analysis, design and mold making, material selection, injection molding process formulation and quality control according to customer needs to ensure the provision of high-quality medical device products that meet customer requirements.
contact us
Try Our Problem-solving Injection Molding Services Now
ISO 13485 Certified factory
ISO 8 Cleanroom
Robust Expertise for Medical Injection Molding
Advanced mold manufacturing capabilities and plastic injection molding equipment
Quick Response within 12 hours
- If you have any questions about products in the medical field, please send us a message(Email: info@ansixtech.com ) at any time and our team will reply to you within 12 hours.